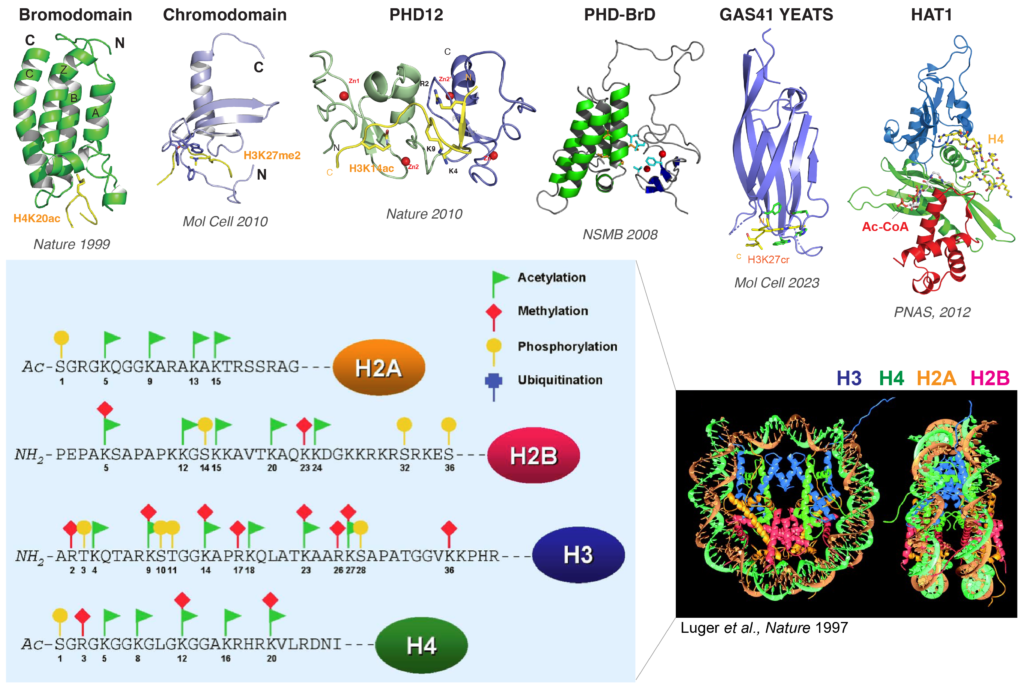
Our research focuses on the fundamental molecular mechanisms of gene transcription within chromatin in both biology and disease. By integrating structural and chemical biology with transcriptomics and proteomics, we investigate the structure, function, mechanisms, and regulation of histone-modifying enzymes and histone reader proteins in transcription.
We discovered the bromodomain as the first histone reader that binds acetylated histones (Nature 1999), a discovery that significantly advanced the understanding of transcription regulation via histones. This work resolved a longstanding question from Vincent Allfrey’s 1964 discovery, showing that histone lysine acetylation recruits effector proteins (trans effects) rather than merely weakening histone-DNA electrostatic interactions (cis effects). The bromodomain-acetyl-lysine interaction is recognized as essential for gene transcription, chromatin remodeling, DNA repair/replication, and cell cycle control.
Follow-up studies (Nature 2010; Mol. Cell 2010) elucidated the structural mechanisms of histone readers, clarifying core principles of histone-regulated transcription. We also demonstrated how specific modifications of histone H3 lysine 27 lead to distinct transcriptional outcomes (Mol. Cell 2023), providing long-awaited experimental evidence supporting C. David Allis’ 2000 “Histone code hypothesis”.
Building on this foundational research, we pioneered bromodomain drug discovery (JACS 2005; Cancer Cell 2014, 2015; PNAS 2017, 2018). These efforts have driven clinical advancements embraced by the pharmaceutical industry, enabling the development of bromodomain-targeted therapies for cancers, inflammatory disorders, and neurodegenerative diseases (Nature Reviews Drug Discovery 2024).